NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons (updated)
NCERT Solutions – Class 11 Chemistry Hydrocarbon :
13.1. How do you account for the formation of ethane during chlorination of methane?
Answer:
Chlorination of methane is a free radical reaction which occurs by the following mechanism:
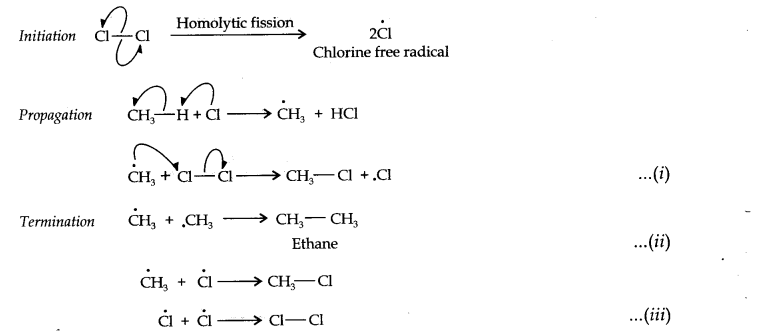
From the above mechanism, it is evident that during propagation step, CH3 free radicals are produced which may undergo three reactions, i.e., (i) — (iii). In the chain termination step, the two CH3 free radicals combine together to form ethane (CH3—CH3) molecule.
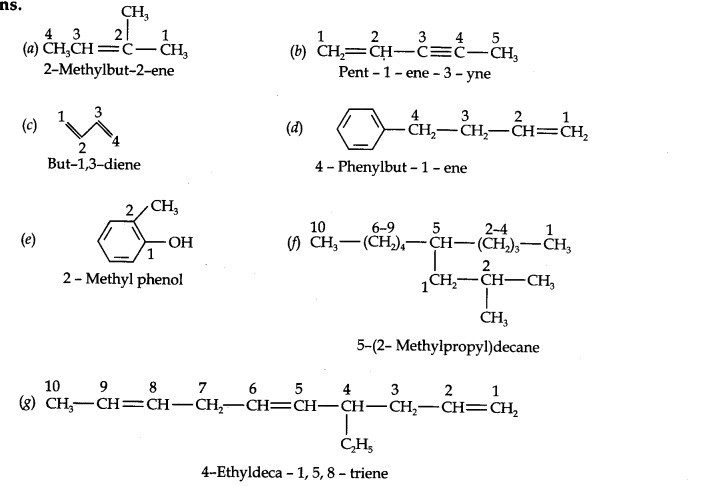
13.2. Write IUPAC names of the following compounds: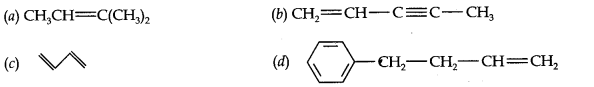
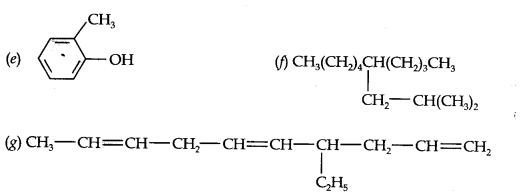
Answer:
13.3. For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated:
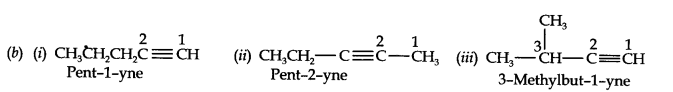
(a) C4H8 (one double bond) (b) C5H8 (one triple bond)
Answer:
(a) Isomers of C4H8 having one double bond are: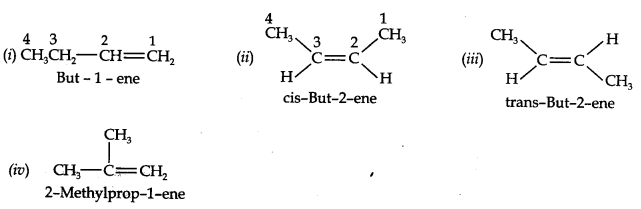
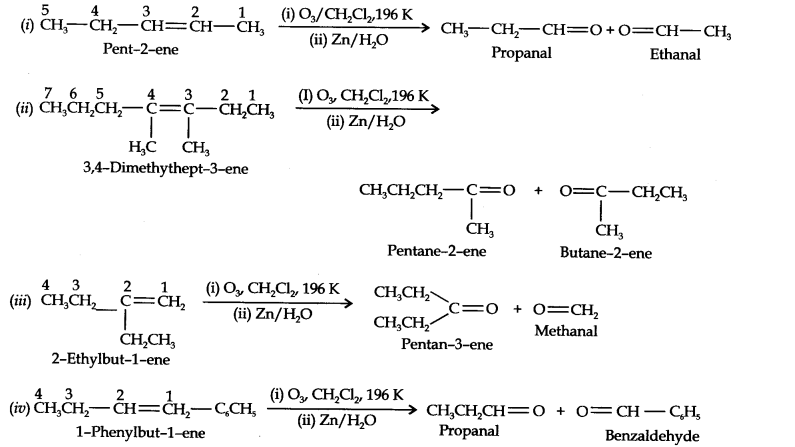
13.4. Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
(i) Pent-2-ene (ii) 3, 4-Dimethylhept-3-ene (iii) 2-Ethylbut-l-ene (iv) 1-Phenylbut-l-ene.
Answer:
13.5. An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3-one. Write the structure and IUPAC name of ‘A’.
Answer:
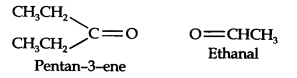
Step 1. Write the structure of the products side by side with their oxygen atoms pointing towards each other.
Step 2. Remove the oxygen atoms and join the two ends by a double bond, the structure of the alkene ‘A’ is
13.6. An alkene ‘A’ contains three C—C, eight C—H, a-bonds, and one C—C n-bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write the IUPAC name of’A’.
Answer:
(i) An aldehyde with molar mass of 44 u is ethanal, CH3CH=0
(ii) Write two moles of ethanal side by side with their oxygen atoms pointing towards each other.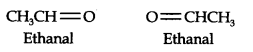
(iii) Remove the oxygen atoms and join them by a double bond, the structure of alkene ‘A’ is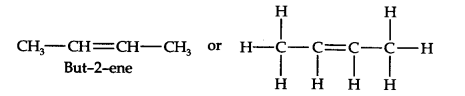
As required, but-2-ene has three C—C, eight C—H a-bonds and one C—C π-bond.
13.7. Propanal and pentan-3-ene are the ozonolysis products of an alkene. What is the structural formula of the alkene?
Answer:
(i) Write the structures of propanal and pentan-3-ene with their oxygen atoms facing each other, we have,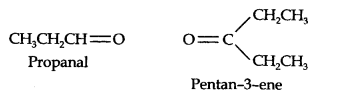
(ii) Remove oxygen atoms and join the two fragments by a double bond, the structure of the alkene is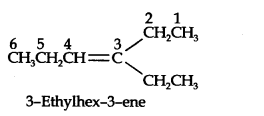
13.8. Write chemical equations for the combustion reaction of the following hydrocarbons, (i) Butane (ii) Pentene (iii) Hexyne (iv) Toluene
Answer:
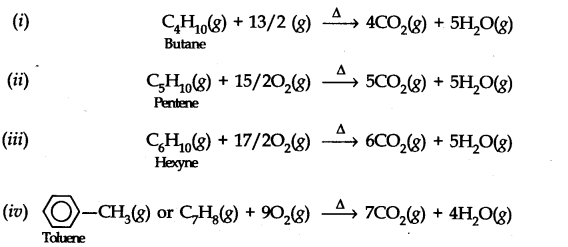
13.9. Draw the cis- and trans-structures for hex-2-ene. Which iosmer will have higher b.p. and why?
Answer:
The structures of cis- and trans-isomer of hex-2-ene are: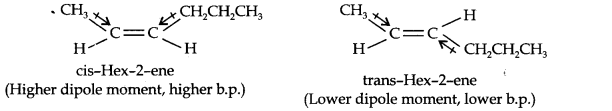
The boiling point of a molecule depends upon dipole-dipole interactions. Since cis-isomer has higher dipole moment, therefore, it has higher boiling point.
13.10. Why is benzene extra-ordinarily stable though it contains three double bonds?
Answer:
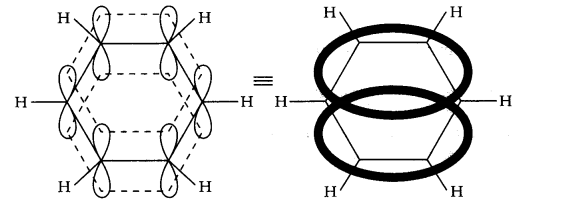
The six electrons of the p-orbitals cover all the six carbon atoms, and are said to be delocalized. As a result of delocalization there formed a stronger n-bond and a more stable molecule.
13.11. What are the necessary conditions for any system to be aromatic?
Answer:
The necessary conditions for a molecule to be aromatic are:
- It should have a single cyclic cloud of delocalised n-electrons above and below the plane of the molecule.
- It should be planar. This is because complete delocalization of n-electrons is possible only if the ring is planar to allow cyclic overlap of p-orbitals.
- It should contain Huckel number of electrons, i.e., (4n + 2) n-electrons where n = 0, 1, 2, 3 etc.
A molecule which does not satisfy any one or more of the above conditions is said to be non-aromatic.
13.12. Explain why the following systems are not aromatic?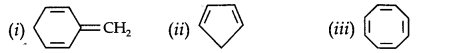
Answer:
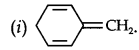
Due to the presence of a sp3-hybridized carbon, the system is not
planar. It does contain six n-electrons but the system is not fully conjugated since all the six n-electrons do not form a single cyclic electron cloud which surrounds all the atoms of the ring. Therefore, it is not an aromatic compound.
(ii)
Due to the presence of a sp3 -carbon, the system is not planar. Further,it contains only four n-electrons, therefore, the system is not aromatic because it does not contain planar cyclic cloud having (4n + 2) n-electrons.
(iii)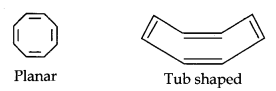
Cyclo-octatetraene is not planar but is tub shaped. It is, therefore, a non-planar system having 8 n-electrons.
Therefore, the molecule is not aromatic since it does not contain a planar cyclic cloud having (4n + 2) n-electrons.
13.13. How will you convert benzene into (i)p-nitrobromobenzene (ii) m-nitrochlorobenzene (iii) p-nitrotoluene (iv) acetophenone?
Answer:
(i) The two substituents in the benzene ring are present at p-positions. Therefore, the sequence of reactions should be such that first an o, p-directing group, i.e., Br atom should be introduced in the benzene ring and this should be followed by nitration. Thus,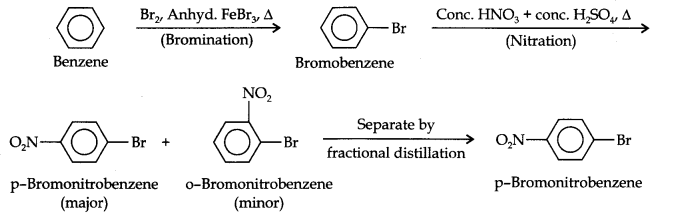
(ii) Here since the two substituents are at p-position w.r.t. each other, therefore, the first substituent in the benzene ring should be a o, p-directing group (i.e., CH3) and then the other group (i.e., NO2) should be introduced.Therefore, the sequence of reactions is: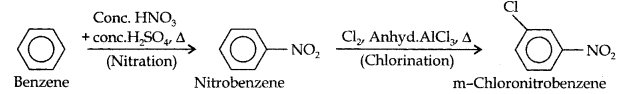
(iii)Here since the two substituents are at m-position w.r.t. each other, therefore, the first substituent in the benzene ring should be a m-directing group (i.e., NO2) and then other group (i.e.,Cl) should be introduced.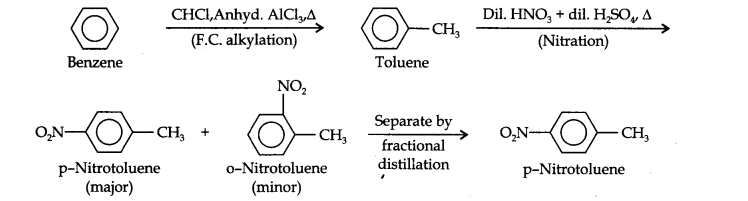
(iv)Acetophenone can be prepared by F.C. acylation using either acetyl chloride or acetic anhydride.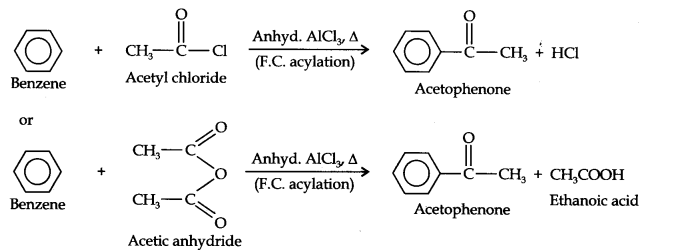
13.14. In the alkane, CH3CH2—C(CH3)2—CH2—CH(CH3)2, identify 1°, 2°, 3° carbon atoms and give the number of H-atoms bonded to each one of these.
Answer:
The expanded formula of the given compound is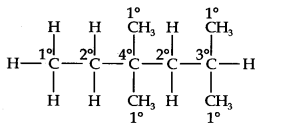
13.15. What effect does branching of an alkane chain has on its boiling point?
Answer:
Branching of carbon atom chain decreases the boiling point of alkane.
13.16. Addition of HBr to propene yields 2-bromopropane, while in presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
Answer:
Addition of HBr to propene is an ionic electrophilic addition reaction in which the electrophile, i.e., H+ first adds to give a more stable 2° carbocation. In the 2nd step, the carbocation is rapidly attacked by the nucleophile Br~ ion to give 2-bromopropane.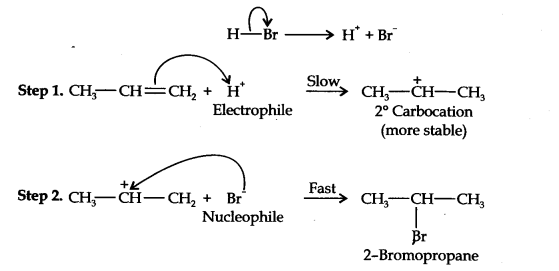
In presence of benzoyl peroxide, the reaction is still electrophilic but the electrophile here is a Br free radical which is obtained by the action of benzoyl peroxide on HBr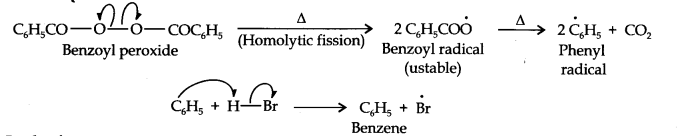
In the first step, Br radical adds to propene in such a way so as to generate the more stable 2° free radical. In the second step, the free radical thus obtained rapidly abstracts a hydrogen atom from HBr to give 1-bromopropane.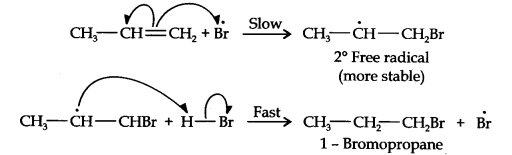
From the above discussion, it is evident that although both reactions are electrophilic addition reactions but it is due to different order of addition of H and Br atoms which gives different products.
13.17. Write down the products ofozonolysis ofl, 2-dimethylbenzene (o-xylene). How does the result support Kekule structure of benzene?
Answer:
o-Xylene may be regarded as a resonance hybrid of the following two Kekule structures. Ozonolysis of each one of these gives two products as shown below: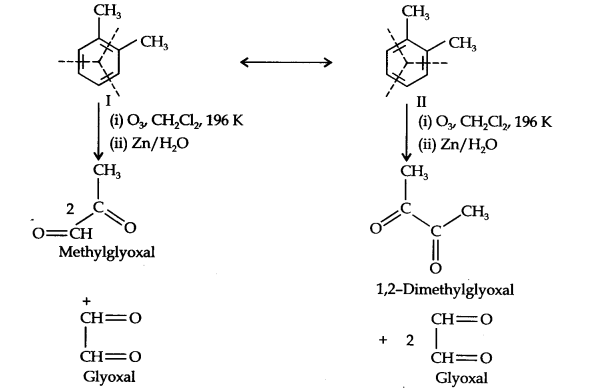
Thus, in all, three products are formed. Since all the three products cannot be obtained from any one of the two Kekule structures, this’ shows that o-xylene is a resonance hybrid of the two Kekule structures (I and II).
13.18. Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also give reason for this behaviour.
Answer:
The hybridization state of carbon in these three compounds is: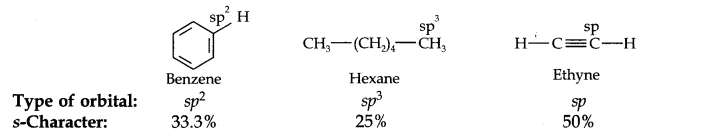
Since s-electrons are closer to the nucleus, therefore, as the s-character of the orbital making the C—H bond increases, the electrons of C—H bond lie closer and closer to the carbon atom. In other words, the partial +ve charge on the H-atom and hence the acidic character increases as the s-character of the orbital increases. Thus, the acidic character decreases in the order: Ethyne > Benzene > Hexane.
13.19. Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Answer:
Due to the presence of an electron cloud containing 6 n-electrons above and below the plane of the ring, benzene is a rich source of electrons. Consequently, it attracts the electrophiles (electron-deficient) reagents towards it and repels nucleophiles (electron- rich) reagents. As a result, benzene undergoes electrophilic substitution reactions easily and nucleophilic substitutions with difficulty.
13.20. How will you convert the following compounds into benzene?
(i) Ethyne (ii) Ethene (iii) Hexane.
Answer: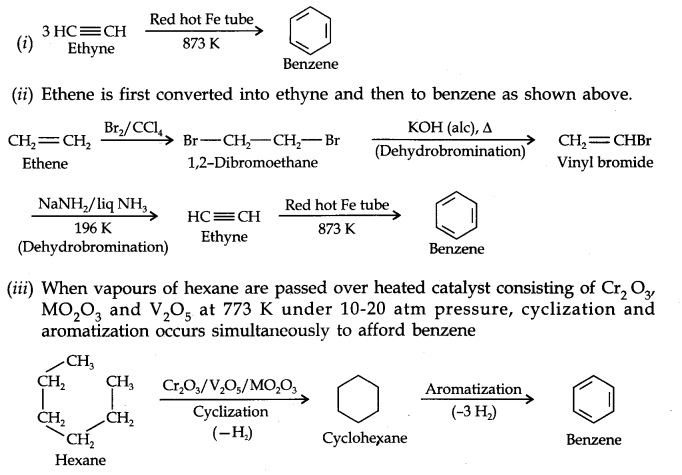
13.21. Write structures of all the alkenes which on hydrogeneration give 2-methylbutane.
Answer: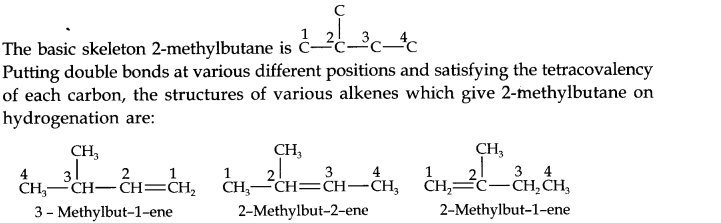
13.22. Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E+.
(a) Chlorobenzene, 2, 4-dinitrochlorobenzene, p-nitrochlorobenzene
(b) Toluene,p—H3C—C6H4—NO2, p—O2N—C6H4—NO2.
Answer:
(a) The typical reactions of benzene are electrophilic substitution reactions. Higher the electron-density in the benzene ring, more reactive is the compound towards these reactions. Since N02 is a more powerful electron-withdrawing group than Cl, therefore, more the number of nitro groups, less reactive is the compound. Thus, the overall reactivity decreases in the order:
Chlorobenzene > p-nitrochlorobenzene > 2, 4-dinitrochlorobenzene
(b) Here, CH3 group is electron donating but N02 group is electron-withdrawing. Therefore, the maximum electron-density will be in toluene, followed by p-nitrotoluene followed by p-dinitrobenzene. Thus, the overall reactivity decreases in the order:
Toluene >p—H3C—C6H4—NO2, p—O2N—C6H4—NO2.
13.23. Out of benzene, m-dinitrobenzene and toluene which will undergo nitration most easily and why?
Answer:
CH3 group is electron-donating while—NO2 group is electron-withdrawing. Therefore, maximum electron density will be in toluene, followed by benzene and least in m-dinitrobenzene. Therefore, the ease of nitration decreases in the order: toluene > benzene > m-dinitrobenzene.
13.24. Suggest the name of another Lewis acid instead of anhydrous aluminium chloride which can be used during ethylation of benzene.
Answer:
Anhydrous Ferric Chloride (FeCl3) is another Lewis acid which can be used.
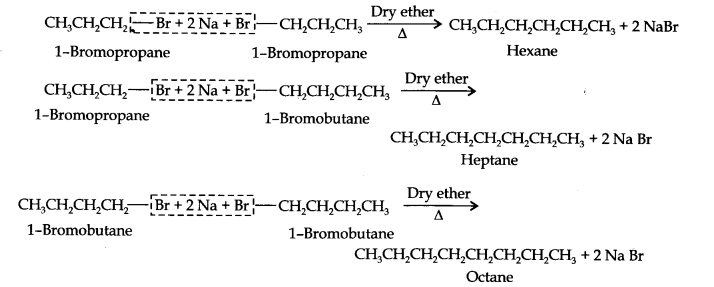
13.25. Why is Wurtz reaction not preferred for preparation of alkanes containing odd number of carbon atoms? Illustrate your answer by taking one example.
Answer:
For preparation of alkanes containing odd number of carbon atoms, a mixture of two alkyl halides has to be used. Since two alkyl halides can react in three different ways, therefore, a mixture of three alkanes instead of the desired alkane would be formed. For example, Wurtz reaction between ‘1-bromopropane and 1-bromobutane gives a mixture of three alkanes i.e., hexane, heptane and octane as shown below: